Introduction to potassium bitartrate instability
Potassium is the most common cause of instability because this metal is a natural constituent of grapes and its presence in wine is usually, therefore, not the result of inadvertent or other addition. Although many potassium salts are soluble in water, potassium bitartrate has limited solubility in aqueous ethanol and the solubility progressively decreases as the alcohol concentration increases. This means that potassium bitartrate precipitation is inevitable as wine processing proceeds and is, therefore, of fundamental importance in winemaking.
Physical characteristics of potassium bitartrate deposits
Potassium bitartrate deposits may be present in amounts ranging from several grams of crystals at the bottom of a bottle to just a few crystals adhering to the cork. The deposit appears variously as small boat-shaped crystals up to large crystalline aggregates of plates resembling shards of glass. It is the latter that causes alarm to the consumer. In red wines the crystals are invariably deeply coloured and dark red, as a result of adsorption of pigments into the crystal lattice.
Factors affecting KHT instability
Experience demonstrates that about half of the tartrate that is present and soluble in grape juice is insoluble in wine. The instability problem is accentuated because the potassium bitartrate may remain in a supersaturated state in the complex wine medium and, in the absence of a stabilisation procedure, will crystallise out after bottling. The crystallisation process depends upon the concentration of potassium bitartrate as well as the presence of crystal nuclei. Factors retarding crystallisation are the presence of certain wine components that may complex with the nuclei and so impede crystal growth. Macromolecules in wine such as proteins, polyphenols and polysaccharides can interact with the bitartrate and inhibit nucleation. Wines differ in their holding capacity for supersaturated potassium bitartrate depending upon the alcohol content, pH, temperature, storage time and interactive effects of various wine components (Zoecklein et al. 1995). Thus, for example, any treatments that cause a change in pH, such as blending or MLF, can affect potassium bitartrate crystallisation and precipitation.
Effects of wine composition on KHT precipitation
The effects of increasing alcohol concentration and decreasing temperature on reducing the solubility of potassium bitartrate are clearly shown from the data in Table 1. This table was produced using data sourced from Berg and Keefer (1958).
Table 1. Solubility of potassium bitartrate (g/L) in alcohol-water solutions1
| Ethanol concentration (% v/v) | |||||
| Temperature (oC) | 0 | 10 | 12 | 14 | 20 |
| 0 | 2.25 | 1.26 | 1.11 | 0.98 | 0.68 |
| 5 | 2.66 | 1.58 | 1.40 | 1.24 | 0.86 |
| 10 | 3.42 | 2.02 | 1.81 | 1.63 | 1.16 |
| 15 | 4.17 | 2.45 | 2.25 | 2.03 | 1.51 |
| 20 | 4.92 | 3.08 | 2.77 | 2.51 | 1.82 |
1. Source of Data: Berg and Keefer (1958)
Precipitation of potassium bitartrate is both influenced by, and has an influence on, the pH and titratable acidity of a wine. These important interactive effects are illustrated in Figure 1.
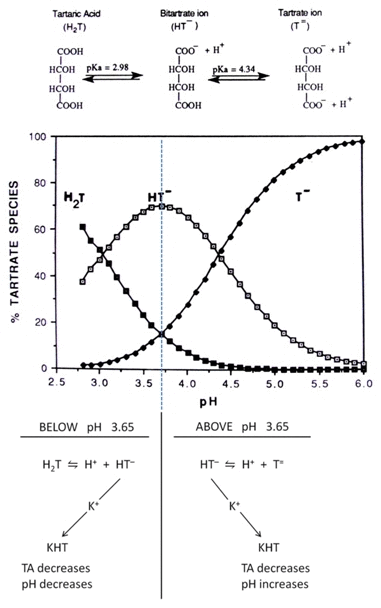
Figure 1. Relative concentration of tartaric acid and species in aqueous solution at different pH values. (Middle and upper part of diagram reproduced from Boulton et al. 1996)
The tartrate distribution diagram in Figure 1 demonstrates the changes that occur on precipitation of potassium bitartrate depending on the initial pH of the wine. Wines with a pH at or below 3.65 show a reduction in pH and titratable acidity after precipitation because of the generation of one free proton per molecule of potassium bitartrate precipitated. At this pH the major equilibrium is between H2T, H+, and HT–, as shown in the left of Figure 1. Precipitation of potassium bitartrate removes HT– from the solution and thus decreases the titratable acidity, while simultaneously shifting the equilibrium to the right and generating more H+ and so lowering the pH.
In contrast, precipitation of potassium bitartrate from a wine with an initial pH above 3.65 still reduces the titratable acidity but now the resulting shift in the major equilibrium, which is between HT–, H+ and T= (as shown on the right in Figure 1) is to the left, removing a proton and increasing the pH. Accordingly, potassium bitartrate precipitation has a positive feed back effect, reducing further the pH of wines initially at pH 3.65 or less, while causing an undesirable increase in the pH of wines initially above 3.65. In both cases the titratable acidity decreases and in the first case the pH may drop by as much as 0.2 pH units.
Once nucleation has occurred further crystal growth follows, resulting in precipitation. In general, a certain level of supersaturation is necessary for adequate nucleation. Some wine components that retard the crystallisation process are proteins, pectins, gums (ie polysaccharides) and polyphenols (Zoecklein et al. 1995). The less complex composition of white wines in relation to reds, means that generally the precipitation of supersaturated potassium bitartrate will occur earlier in the life of a white wine than from a red. Important exceptions to this generalisation are Botrytis-affected sweet whites which are notoriously difficult to stabilise. Glucans from Botrytis infection interfere strongly with the crystallisation process and are capable of extensively delaying and even preventing the precipitation of potassium (Ribéreau-Gayon et al. 2000).
Effects of time and temperature on potassium bitartrate precipitation
A lengthy period of post-fermentation processing (eg wood maturation for reds and for some full-bodied whites) will lead to a natural precipitation of KHT, and these wines will, therefore, enjoy some degree of natural self-stabilisation. Nevertheless, tests should be done to ensure the stability of these wines. Other wines, such as light-bodied whites, which do not spend as much time after fermentation and before bottling, will retain most or all of their unstable potassium bitartrate. These wines must be fully stabilised and stability tested as part of their finishing.
Accordingly, each wine because of its unique composition will have a particular level of potassium bitartrate supersaturation at a given temperature. Decreasing the temperature of the wine alone, or in combination with seeding through the addition of potassium bitartrate crystals, greatly enhance the processes of crystallisation. Such cold stabilisation procedures are made further effective after bentonite fining the wine to remove proteins and reduce the levels of other components that might interfere with the precipitation process (Zoecklein et al. 1995, Boulton et al. 1996).
Principles behind stabilising wines for potassium bitartrate
Fortunately, the potential for potassium bitartrate to precipitate from a wine can be predicted, with varying degrees of reliability, by a number of tests. These include:
- a refrigerated or cold stability test by storage of a sample of the wine at a specific temperature/time regimen and then inspection for signs of crystal formation
- inducing crystal formation by freezing and thawing the wine
- the determination of concentration product (CP) values
- measurement of the change in conductivity of a refrigerated sample of the wine after seeding with potassium bitartrate crystals.
The AWRI has adopted the refrigerated cold stability test as a recommended standard. In an evaluation of the four tests above, as applied to Australian wines, the reliability of the cold stability test was demonstrated (Leske et al. 1996). Importantly, cold stability determined at -4°C for three days was found to relate well to actual deposition of potassium bitartrate crystals in a wide range of commercial red and white wines. Also, the test requires little capital equipment. Having established the potential instability of a sample, the bulk wine is held at 0 to -2°C to precipitate potassium bitartrate crystals in the holding tank until a repeat cold test demonstrates that the wine is stable.
All stabilisation and associated stability tests must be done as the final procedure before bottling. This is because any alteration in the composition of a wine, eg. change in pH or change in protein content, will change the susceptibility of the wine to precipitate KHT. Therefore, all blending, MLF, bentonite fining, acid adjustments etc, should be complete before the wine is given its final KHT stabilisation and stability check.
Because of the interactive effects of pH and potassium bitartrate precipitation illustrated in Figure 1, it is important that the initial pH of a wine be below 3.65 during cold stabilisation and during vinification when some precipitation may occur naturally. In this way the decrease in titratable acidity will be coupled with a lowering of the pH which is advantageous to the winemaker.

