Heat instability of wine is generally due to precipitation of unstable grape proteins. This is most commonly relevant to white table wines, but may also be a consideration for rosé, sparkling and some red wines. Most wineries remove unstable proteins from white wine before bottling by fining with bentonite. The bentonite reduces the amount of protein in the wine to levels where protein will not precipitate over time; however, several factors can affect this stability and it is important to test heat stability prior to bottling. This page gives a brief summary of the procedures and equipment for some commonly used tests for heat stability of wines.
Heat test
Filter two 20 mL samples of the wine through a 0.45 µm membrane into two 25 mL screw-capped test tubes. Examine both samples using a strong light source to ensure that the wine is brilliantly clear after the filtration . If a turbidimeter is available, an alternative option is to measure initial turbidity of the filtered samples. A turbidity reading of less than 2.0 NTU is recommended. Place one of the tubes in a water bath pre-heated to 80°C, ensuring that the entire volume of wine in the tube is immersed in the water bath, but the top of the tube is not covered by water. Leave the other tube (the control sample) at approximately 20°C. Heat the sample at 80°C for two hours. While six hours has been typical industry practice for the heat test, recent research has shown that two hours of heating time is sufficient. After heating, remove the heated tube from the water bath and leave it to return to room temperature for 3 hours. A minimum of 3 hours cooling is required for the heated proteins to aggregate and precipitate, so chilling on ice or under cool water to quickly lower temperature is not recommended. If using the traditional method of heating for 6 hours, cool overnight. Be consistent with the selected heating time (2 or 6 hours) and cooling time (3 hours or overnight) for reproducible results.
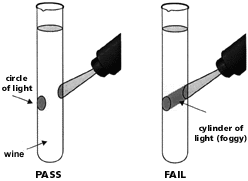
After the cooling period, examine the heated sample for any haze by holding it against the strong light source and then compare it against the unheated control. If a haze is observed in the heated sample that is not present in the unheated control, the wine is heat unstable and therefore susceptible to the formation of protein hazes. If the heated wine remains clear, the wine is heat stable and therefore not likely to form a protein haze or deposit. Because the observation of the haziness of the heated sample is subjective, sometimes conflicting results can arise between different laboratories or different analysts. To avoid this, it is best to carry out the test in the most stringent way possible. This can be achieved by using a focusable light equipped with an adjustable iris diaphragm, which produces a narrow and focused beam of light, allowing very faint hazes to be detected. If the beam of light is only visible as it enters and leaves the tube, the sample passes (i.e. is considered heat stable), but if the beam can be seen through the sample, it fails and the wine is considered heat unstable. It is advisable to use two people to make this qualitative assessment.
A turbidimeter can be used for a more objective comparison of the turbidity in the two samples. In this case, wines that exhibit a turbidity increase of greater than a given criterion for nephelometric turbidity units (NTU) after heating, as compared with the unheated control, can be considered to have failed the heat stability test. Some laboratories use a criterion of 0.5 NTU, but other practitioners in industry have indicated that a more reasonable criterion is less than (not equal to) 2.0 NTU.
Recommended heating time at 80°C and subsequent cooling times at ambient temperature are shown below. Note that these recommendations are the same for each wine matrix.
| Matrix | Heating time | Cooling time | Turbidity difference for pass result |
| White wine | 2 hours | 3 hours | < 2.0 NTU |
| Rosé wine | 2 hours | 3 hours | < 2.0 NTU |
| Red wine | 2 hours | 3 hours | < 2.0 NTU |
*Some laboratories require turbidity differences of <0.5 NTU for a pass result.
Equipment:


Membrane filtration and containers for wine samples (e.g. 25 mL screw capped glass tubes or turbidity tubes)


Water bath or heating mantle

Visual assessment using a light source

Turbidimeter measurement
Reagents: none
Services: wash-up area
Space required: minimal bench space
Commercial reagents
A number of reagents for testing heat stability are available commercially. These include products such as Bentotest® and Proteotest®. This type of chemical test is rapid and does not require heating of the test samples; however, they are considered to be more stringent than the heat test (Rankine 1989) and can result in much larger additions of bentonite than are actually required. To use these reagents, follow the product instructions and add the required amount of reagent to the required volume of wine. Examine the solution by eye for the presence of haze. As in the case of the heat test, a turbidimeter can be used for more objective assessment of the turbidity in the sample.
Equipment required: test tubes, filtration equipment, turbidimeter (optional)
Reagents: commercially available reagent
Services: wash-up area
Space required: minimal bench space
Additional resources
- Predicting heat stability of wine: the heat test revisited (AWRI webinar 16 August 2017)
- Protein stability (AWRI fact sheet)
- Iland, P.; Ewart, A.; Sitters, J.; Markides, A.; Bruer, N. 2000. Techniques for chemical analysis and quality monitoring during winemaking. Campbelltown, SA: Patrick Iland Wine Promotions: 80-81.
- McRae, J.M., Barricklow, V., Pocock, K.F., Smith, P.A. 2018. Predicting protein haze formation in white wines. Aust. J. Grape Wine Res. 24(4): 504-511.
- Pocock, K.F.; Rankine, B.C. 1973. Heat test for detecting protein instability in wine. Aust. Wine Brew. Spirit Rev. 91: 42-43.
- Rankine, B.C. 1989. Making good wine. Melbourne, Vic: Macmillan Pty Ltd: 313.
- Rankine, B.C.; Pocock, K.F. 1976. Wine stability tests for quality control. Aust. Wine Brew. Spirit Rev. 96(12): 24-25.
- Toland, T.M.; Fugelsang, K.C.; Muller, C.J. 1996. Methods for estimating protein instability in white wines: a comparison. Am.. J. Enol. Vitic. 47(1): 111-112.
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. 1999. Production Wine Analysis. New York: AVI Van Nostrand Reinhold: 469-473.
Contact the AWRI information services team to request copies of resources not available online.

