In natural environments, microorganisms usually exist as mixed populations. However, if bacteria, yeast and other microorganisms are to be studied, characterised, or identified it is important to be able to work with them in such a way that the sample, the surrounding environment, or the operator is not contaminated. There must also be a method of transferring growing organisms from one medium to another without introducing any unwanted outside contaminants. This method of preventing unwanted microorganisms from gaining access to samples or cultures is termed aseptic technique. Aseptic transfers are often conducted in a laminar flow cabinet that has surfaces that can be sterilised before use with alcohol swabbing and ultraviolet light, and sterile filtered air is supplied to maintain postive air pressure in the working area of the cabinet to prevent settling of any potential contaminants.
The procedure for aseptically transferring microorganisms is as follows:
- Sterilise the inoculating loop.
The inoculating loop is sterilised by passing it at an angle through the flame of a gas burner until the entire length of the wire becomes orange from the heat. In this way all contaminants on the wire are incinerated. Never lay the loop down once it is sterilised, or it may again become contaminated. Allow the loop to cool a few seconds before contacting the inoculum to avoid killing the microorganisms. - Remove the inoculum.
- Removing the inoculum from a broth culture (microorganisms growing in a liquid medium):
- hold the culture tube in one hand and in your other hand, hold the sterilised inoculating loop as if it were a pencil.
- remove the cap of the pure culture tube with the little finger of your loop hand and ensure that the open end of the cap is facing downward to minimmise the risk of airborne contaminiants settling in the cap. Never lay the cap down or it may become contaminated.
- very briefly flame the lip of the culture tube. This creates a convection current which forces air out of the tube and prevents airborne contaminants from entering the tube.
- keeping the culture tube at an angle, insert the inoculating loop and remove a loopful of inoculum.
- again flame the lip of the culture tube.
- replace the cap.
- Removing the inoculum from a plate culture (microorganisms growing on an agar surface in a petri plate):
- lift the lid of the culture plate slightly and stab the loop into the agar away from any growth, to cool the loop.
- scrape off a small amount of the organism and close the lid.
- Transfer the inoculum to the sterile medium.
- Transferring the inoculum into a broth tube:
- pick up the sterile broth tube and remove the cap with the little finger of your loop hand. Do not set the cap down and ensure that the open end is facing downward.
- briefly flame the lip of the broth tube.
- place the loopful of inoculum into the broth, and withdraw the loop. Do not lay the loop down!
- again flame the lip of the tube.
- replace the cap.
- resterilise the loop by placing it in the flame until it is orange. Now you may lay the loop down until it is needed again.
- Transferring the inoculum to an agar plate:
- lift the edge of the lid just enough to insert the loop.
- streak the loop across the surface of the agar medium using the following steps:
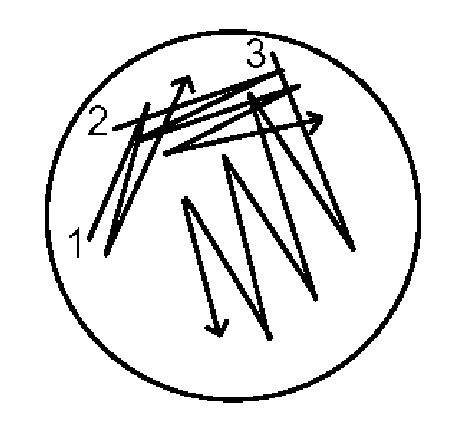
- Sterilize a wire loop by heating it until red hot in a flame; allow it to cool for several seconds. Test for coolness by touching the agar at the edge of the plate.
- Pick up a loopful of liquid inoculum or bacterial growth from the surface of an agar plate and, starting about 25 mm in from the edge of the plate (1), streak lightly back and forth with the loop flat, making close, parallel streaks back to the edge of the plate.
- Sterilize the loop and cool again, then with the edge of the loop, lightly make another set of nearly parallel streaks about 3 mm apart, starting from the inoculated area (2) to one side of the uninoculated area, so that about 1/2 the plate is now covered.
- Flame and cool the loop again, and make another set of streaks starting from (3) perpendicular to and crossing the second set of streaks, but avoiding the first set.
Every aseptic procedure in the microbiological laboratory will be done using similar techniques, with modifications as necessary.

