This page provides information on taints, faults and flavours commonly encountered in wine. Use the links below to jump to the characters that are of interest.
Wine faults:
Oxidation-type faults
Oxidation
Oxidation, particularly of white wines, was a common fault in Australian white wines 40 years ago when our table wine technology was reasonably primitive, compared to that of today. Oxidation is much less common today with the application of refrigeration, inert gas blanketing during the production and packaging operations and effective sulfur dioxide management. The oxidation flavour is due to multiple compounds including a range of aldehydes.
Some wines are more sensitive to oxidation than others. White wines made from the ‘floral’ varieties such as Riesling are very prone to oxidation, whereas red wines can withstand significant oxidation during handling due to the higher content of phenolic compounds, which are natural antioxidants. The sensory characteristics of oxidation range from a dulling of the aroma, to ‘cardboard’, ‘straw’ and ‘hay-like’ aromas, to ‘sherry-like’ and ‘madeirised’. In extreme cases a ‘wet wool’, ‘wet dog’, or ‘varnish-like’ aroma can be evident. Of course for some wine styles, such as sherry, oxidation is deliberately encouraged.
Acetaldehyde
The sensory threshold for acetaldehyde ranges from 100-125 mg/L. Immediately after fermentation, table wines generally have acetaldehyde levels below 75 mg/L. However, above 125 mg/L acetaldehyde can impart odours described as ‘over-ripe bruised apples’, ‘stuck ferment’ character or ‘sherry’ and ‘nut-like’ characters. Yeast can oxidise ethanol to acetaldehyde under oxidative conditions. Therefore ullaged tanks can lead to surface yeast infection where acetaledehyde is produced (note that high levels of acetic acid and ethyl acetate may also be produced under these conditions). Ethanol represents the primary source of carbon in aerobic film-yeast growth.
Acetaldehyde levels increase as wines age due to chemical oxidation of ethanol. As acetaldehyde is also an intermediate in the bacterial formation of acetic acid and under low-oxygen conditions and/or alcohol levels greater than 10% v/v, acetaldehyde tends to accumulate instead of being oxidised to acetic acid.
Apart from chemical and microbiological formation, winemaking practices can influence the level of acetaldehyde present in wine; addition of SO2 during fermentation can increase the concentration of acetaldehyde, as can increases in pH and fermentation temperature.
Volatile acidity
Volatile acidity (VA) is a term that probably represents the wine industry’s first measure of wine quality, although in a negative sense. As a result, the measure of volatile acidity is still prominent in the wine regulations of most countries, even though the components of volatile acidity represent no threat to health, and the amount of volatile acidity tolerated will vary with the style of wine and the individual. The legal maximum content of volatile acidity in Australian wines, excluding SO2 and expressed as acetic acid, is 1.5 g/L.
Volatile acidity is a measure of the low molecular weight, or steam-distillable, fatty acids in wine, with by far the major acid being acetic acid (>93%) (click here for more information). However, other contributors to the chemical measure of VA include carbonic acid (from carbon dioxide), sulfurous acid (from SO2), as well as lactic, formic, butyric and propionic acids. Note that sorbic acid is also steam-distillable and should be taken into account if it has been added to wine (usually as potassium sorbate).
Generally perceived as the odour of vinegar, volatile acidity or acetic acid has a reported aroma threshold in wine to be as low as 0.1 – 0.125 g/L, depending on the style of wine and the individual. However, the concentration at which the acid is regarded as detrimental is usually greater than 0.7 g/L. The volatile acidity is more easily detected if a small amount of ethyl acetate is also present, and is some cases ethyl acetate aroma can dominate.
During alcoholic fermentation, yeast produce small amounts of acetic acid, and the VA of a sound wine immediately after fermentation is usually in the range 0.1 – 0.4 g/L. However, native or wild yeasts such as Hansenula and Kloeckera can produce high concentrations of acetic acid before and during the early stages of fermentation. In addition, Brettanomyces can produce elevated levels of VA when grown under aerobic conditions. Some strains of Saccharomyces can also produce large amounts of acetic acid when placed under stress, i.e. during low or high temperature fermentations, during fermentation of high sugar musts, when available nitrogen is low or when the pH is low (i.e. >3.2). In addition, the AWRI has found that addition of vitamin mixtures to white grape juice fermentations increased the acetic acid concentration in the wines. The increase was highly variable and depended on the grape juice, with the magnitude of the increase varying from 30% to 375%. Nicotinic acid and thiamine were the vitamins which most affected the increase in VA.
Increased levels of acetic acid in stored wines are usually attributable to growth of acetic acid bacteria (generally of the genus Acetobacter). These bacteria convert alcohol to acetic acid in the presence of oxygen. In fact, until Pasteur’s work in the mid-19th century, a high proportion of wines spoiled through this mechanism before they could be consumed. Acetic acid bacteria are often isolated from red wines analysed at the AWRI, however, spoilage problems related to the growth of these bacteria only occur when the wines are exposed to air. In addition to lactic acid and carbon dioxide, heterolactic lactic acid bacteria (LAB) might also produce elevated amounts of acetic acid when growing in the presence of glucose. Small amounts of acetic acid might also be produced during wine storage in new oak barrels due to hydrolysis of acetyl groups in the wood hemicellulose. In addition, it has been reported that acetic acid can result from the reaction of hydrogen peroxide, generated from coupled oxidation of wine phenolics, with ethanol to generate acetaldehyde, which is in turn oxidised to acetic acid.
Ethyl acetate
Various acetate esters, especially ethyl acetate, can contribute to the sensory perception of volatile acidity, as indicated above. Ethyl acetate is perceived as the odour of nail polish remover and has a reported sensory threshold of 12 mg/L. Ethyl acetate is the major ester produced by yeast and at low levels can contribute ‘fruity’ aroma properties and add complexity to wine. The concentration of ethyl acetate ranges from about 30 – 60 mg/L in ‘normal’ wines, to about 150 – 200 mg/L in defective wines.
Factors that can influence ethyl acetate formation by yeasts include the yeast strain employed, temperature of fermentation, the amino nitrogen content of the juice and sulfur dioxide levels. As with acetic acid, native or wild yeasts such as Hansenula and Kloeckera can produce high concentrations of ethyl acetate before and during the early stages of fermentation. Ethyl acetate is also produced by acetic acid bacteria and is related to dissolved oxygen levels in the wine. It has been reported that growth of acetic acid bacteria under conditions of low oxygen tension can lead to higher levels of ethyl acetate.
Mousiness
Mousy taint is an off-flavour reminiscent of caged mice or sometimes cracker biscuit, and in sensitive individuals renders the wine undrinkable. The taint is generally perceived late on the palate or after the wine has been swallowed or expectorated and usually takes a few seconds to build. It tends to linger and leave a most obnoxious taste in the mouth for some time. If you move quickly to the next wine in a line-up, you might miss a mousy wine. Mousy taint is rarely detected by sniffing because the compounds involved are not volatile at wine pH. Note that there is considerable variation in the sensitivity between individuals to the taint.
The compounds responsible for ‘mousiness’ are the N-heterocyclic volatile bases 2-acetyltetrahydropyridine (ACTPY), which is the main compound responsible, 2-ethyltetrahydropyridine (ETPY) and 2-acetylpyrroline (ACPY) (Grbin et al 1996). The sensory thresholds of these compounds in water and the ranges found in wine are given in Table 1.
Table 1. Sensory thresholds of the compounds responsible for ‘mousiness’ in water and ranges found in wine.
| Compound |
Threshold in water (µg/L) |
Range in wine (µg/L) |
| ACTPY | 1.6 | 4.8 – 106 |
| ETPY | 150 | 2.7 – 18.7 |
| ACPY | 0.1 | trace – 7.8 |
Usually of microbial origin, most strains of lactic acid bacteria (LAB) being capable of producing the taint, particularly the heterofermentative species. These include Lactobacillus hilgardii, Lactobacillus plantarum, Lactobacillus brevis, and Oenococcus oeni (Cosello et al 2001). The yeast Dekkera/Brettanomyces may also be capable of producing mousy compounds. In addition to microbial origin, empirical observation has shown that some wines develop mousy taint when exposed to air or oxygen. The mechanism by which oxidation enhances mousy taint is currently unknown. There is no satisfactory method to remove mousy off-flavour, which is more likely to occur in wines with low concentrations of SO2 and low acidity.
The AWRI observed an increased incidence of mousy wines during the 1990s when winemakers moved to a lower sulfur dioxide regime for the production of red wines in particular. In the 2010s, another increase in mousy wines was observed when some winemakers experimented with extended lees ageing, high pH with minimal sulfur dioxide (SO2), oxidative ageing and minimal clarification or filtration in white wines. For more information, refer to this Ask the AWRI column on avoiding mousy off-flavours. Such regimes demand a fastidious approach to cellar hygiene to prevent unwanted microbial growth and the possible formation of ‘mousiness’. The AWRI has recommended to winemakers that they should work at between 50 and 80 mg/L total sulfur dioxide (more for wines of high pH) for red wine production if they have any doubts about their cellar sanitation.
Reductive wine faults
A range of volatile sulfur compounds can form in wine, resulting in a variety of unwanted ‘reductive’ sensory characters, as detailed below. For information on how to manage these compounds during winemaking and prior to packaging, visit the Removal of volatile sulfur compounds page.
Hydrogen sulfide
Most winemakers will be familiar with the aroma of hydrogen sulfide (H2S) or ‘rotten egg gas’. The detection threshold of H2S in wine is about 1 – 2 µg/L (parts per billion) and it has been reported that concentrations below this threshold might play a role in wine complexity. The various forms of sulfur (e.g. sulfate, sulfite and sulfur-containing amino acids) are important for yeast biosynthesis. During alcoholic fermentation, yeast will excrete hydrogen sulfide into the fermenting juice when placed under stress, e.g. when the yeast starts to run out of nitrogen. Australian juices can be low in nitrogen and winemakers often supplement the juice with a soluble nitrogen source, such as diammonium phosphate.
Hydrogen sulfide can be produced in excess by yeast during fermentation due to the presence of elemental sulfur on grape skins (from sulfur sprays), inadequate levels of free α-amino nitrogen (FAN), added SO2, a deficiency of B-complex vitamins (pantothenic acid or pyridoxine), unusually high levels of cysteine in the juice or a high concentration of metal ions. The production of H2S can also be yeast strain dependent.
Winemakers minimise the formation of excess H2S in white wines by either settling, centrifuging or filtering the must before fermentation, which removes high-density solids which might contain elemental sulfur. Some winemakers remove excess H2S from red wines by aerating at the first racking, thus volatilising the H2S. Aeration might also oxidise H2S to elemental sulfur (S), however, the S precipitate must be removed (centrifugation or filtration) otherwise it might later reform H2S, when conditions become favourable for reduction. Note that SO2 can also convert H2S to S. Many winemakers remove objectionable H2S in red and white wines by fining with copper sulfate (CuSO4). Copper sulfate reacts with H2S to form copper sulfide, which is highly insoluble. However, careful laboratory trials should precede any CuSO4 additions to bulk wine, as an instability can result if the copper concentration in the wine exceeds approximately 0.5 mg/L (even lower in some wines).
Unwanted sulfhydryls
Sulfhydryls are compounds that contains a -SH group. These compounds are also known as ‘thiols’ or ‘mercaptans’, with aromas variously described as ‘cabbage’, ‘garlic’, ‘onion’ and ‘rubber’ and many other colourful terms. Their presence in wine above the threshold is generally regarded as a defect, however, the odour of these sulfur compounds is important to many foods.
It is thought that ethyl mercaptan (ethanethiol) is probably formed in wine by the direct chemical reaction between H2S and ethanol. Methyl mercaptan (methanethiol) might be formed in a similar way, by the direct chemical reaction between H2S and methanol. However, it is known that methyl mercaptan is formed directly as a result of yeast metabolism, therefore, it is best to remove H2S before it reacts further to form thiols.
The aroma of ethyl mercaptan is described as ‘onion-like’ and ‘rubber-like’. The sensory threshold for ethyl mercaptan is 1.1 µg/L. The aroma of methyl mercaptan is described as ‘rotten eggs’ and ‘cabbage’. The sensory threshold for methyl mercaptan is 0.02 – 2.0 µg/L.
Disulfides
Sulfhydryls such as ethanethiol (ethyl mercaptan) and methanethiol (methyl mercaptan) can be rapidly oxidised to diethyldisulfide (DEDS) and dimethyldisulfide (DMDS), respectively. The aroma of DMDS is described as ‘onions’ and ‘cooked cabbage’ and its sensory threshold is 29 µg/L. The aroma of DEDS is described as ‘burnt rubber’ and ‘garlic’ and its sensory threshold DEDS is 4.3 µg/L.
If a wine containing methanethiol and ethanethiol is aerated to remove a suspected hydrogen sulfide fault, these can be oxidised to DMDS and DEDS, which do not react with copper and therefore cannot be removed by copper fining. Removal of DMDS and DEDS requires the creation of reducing conditions, by the addition of ascorbic acid and SO2, in order to reduce these compounds back to the reactive species (methanethiol and ethanethiol), which may then be removed by treatment with copper.
To do this, first ensure there is >30 mg/L free SO2 in the wine. For white wines, add 10 mg/L ascorbic acid and then another 10 mg/L ascorbic acid the following day. For red wines, add 2 mg/L ascorbic acid and then another 2 mg/L ascorbic acid the following day. Wait another 24 hours for the ascorbic acid to react with any free oxygen and to allow disulfides to be reduced back to mercaptans. A copper fining trial can then be performed on this treated wine to determine the appropriate copper addition rate to react with and remove the methanethiol and ethanethiol.
Dimethyl sulfide (DMS)
Dimethyl sulfide (DMS) is one of the major compounds found in aged wines and is formed during the maturation of wine in the bottle, however, the mechanism of formation of DMS is not clearly known. At low concentrations it might contribute toward the body of aged white wines and has a ‘vegy’ or ‘blackcurrant’ character. At higher concentrations, the aroma of DMS is described as a fault and is described as ‘asparagus’, ‘cooked corn’, ‘cooked tomato’ or ‘molasses’. The sensory threshold for DMS is between 30 – 60 µg/L. As dimethyl sulfide does not bind to copper it can be difficult to remove the aroma, however, removal might be possible by sparging with nitrogen or by using reverse osmosis.
Additive-related faults
Sulfur dioxide (SO2)
Sulfur dioxide is one of two preservatives permitted for use in wine production in most winemaking countries the other preservative is sorbic acid. Most countries set a legal maximum for the total sulfur dioxide content of wine. In Australia, it is 250 mg/L in total sulfur dioxide in products containing less than 35 g/L sugars, or 300 mg/L in total of sulfur dioxide for other products.
Winemakers add SO2 to wine to minimise the effects of oxidation and also to inhibit microbiological activity. Winemakers refer to three categories of SO2: free, bound and total. The free SO2 is defined as the sum of the unreacted ionic forms, which are the molecular, bisulfite and sulfite forms. The bound SO2 involves the portion of the bisulfite form which binds with particular wine components (to form bisulfite addition compounds) and which can be released by hydrolysis and/or by heat and distillation. Total SO2 represents the sum of the free and bound fractions (Sneyd et al. 1993). The sulfite form (SO3=) of SO2 in wine is the form that reacts with molecular oxygen, however, at wine pH this form is the least abundant. At wine pH, the most abundant forms of SO2 are the molecular (SO2) and bisulfite (HSO3) forms. Neither of these forms reacts with oxygen, however, molecular SO2 does react quickly with hydrogen peroxide (H2O2). This reaction is responsible for the removal of H2O2 produced by the reaction of oxygen with polyphenols and the retardation of acetaldehyde formation and browning in wines. In addition, it is the molecular form that is responsible for the anti-microbial effect of SO2 and is the form that we can smell when too much is added.
Sulfur dioxide has a pungent penetrating aroma which reacts strongly with receptors in the nose causing sneezing and often a choking sensation – a high content of free sulfur dioxide can be life threatening to a small proportion of asthmatics. A content of free sulfur dioxide up to 15 mg/L has no adverse sensory effect.
Diacetyl
Diacetyl (2,3-butane dione) can be produced by both yeast and bacteria, and at low levels (1 – 4 mg/L) can add complexity to a wine by imparting ‘buttery’ or ‘butterscotch’ characters. At high levels (>5 mg/L) the aroma might be considered objectionable, such that the wine might be regarded as defective.
The amount of diacetyl produced by yeasts is typically less than 1 mg/L, which is below the sensory threshold. Bacterial production of diacetyl during malolactic fermentation (MLF) represents the primary source of this compound and arises mainly from catabolism of citric acid. Under winemaking conditions, this generally occurs after all of the malic acid has been converted.
Formation of diacetyl is dependent on a number of factors, including the bacterial strain, the oxygen (O2) tension of wine (increase in O2 concentration favours the oxidation of α-acetolactate to yield diacetyl), the citric acid concentration and temperature.
Geranium
Geranium aroma is caused by the ether 2-ethoxyhexa-3,5-diene (Crowell and Guymon 1975), which has an odour reminiscent of crushed geranium leaves. It is formed from the metabolism of sorbic acid by lactic acid bacteria (LAB). Sorbic acid (another wine preservative) is a short-chained unsaturated fatty acid and is widely used as a anti-fungal agent in sweet wines at bottling. While sorbic acid is generally effective in the inhibition of Saccharomyces yeast, it has little activity toward Dekkera/Brettanomyces and Zygosaccharomyces yeast, or LAB or acetic acid bacteria.
Edinger and Splittstoesser (1986) found that numerous strains of Oenococcus oeni were able to metabolise sorbic acid to sorbic alcohol (2,4-hexadien-1-ol), the precursor to the geranium character, while various strains of Lactobacillus and Pediococcus did not produce sorbic alcohol. Once 2,4-hexadien-1-ol (sorbic alcohol) is formed by the bacteria, the compound rearranges under the acidic conditions of wine to 3,5-hexadiene-2-ol, which in turn reacts with ethanol to form the volatile ether 2-ethoxyhexa-3,5-diene, which is responsible for the geranium off-odour (Crowell and Guymon 1975).
Using gas chromatography-olfactometry, gas chromatography-mass spectrometry and dilution techniques, Chisholm and Samuels (1992) found the odour detection threshold of 2-ethoxyhexa-3,5-diene to be <1 ng/L, which indicates this compound is extremely potent. Given its very low aroma threshold, the geranium off-odour from 2-ethoxyhexa-3,5-diene is practically impossible to remove. The odour remains after carbon treatment, is present in spirits after distillation and is still perceived after significant dilution (Ribéreau-Gayon et al. 2006). Consequently, it is imperative that wine conditions (the combination of pH, SO2 and ethanol concentrations) are such that growth of lactic acid bacteria is strongly inhibited and that sorbic acid is not used to treat red wines.
Brettanomyces faults
The volatile phenol 4 ethylphenol is the major spoilage compound associated with the growth of Dekkera/Brettanomyces yeast in wine. This compound imparts a ‘Band aid®’, ‘medicinal’ or ‘pharmaceutical’ character to wine. A concentration of 425 µg/L of 4-ethylphenol has been reported to have a negative impact on the quality of many wines (Chatonnet et al 1992, 1993). Recent Australian AWRI sensory studies has shown the aroma threshold in Australian wine to be 368 µg/L in a neutral red wine (Figure 1) (Curtin et al. 2008).
Figure 1. Aroma thresholds of 4-ethyl guaiacol (4-EG), 4-ethyl phenol (4-EP), and 4-ethyl catechol (4-EC) in three different Australian Cabernet Sauvignon wines.
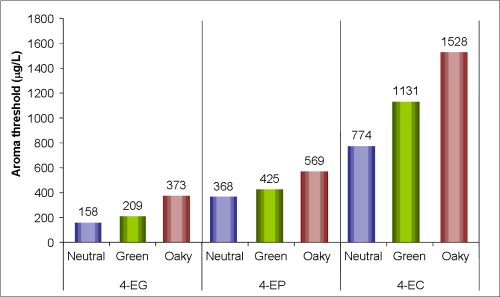
Evidence from sensory assessments and analyses conducted at the AWRI show that the sensory perception threshold of 4-ethylphenol depends very much on the style and structure of the wine. For example, the aroma threshold of 4-ethylphenol in a full bodied red wine with intense methoxypyrazine green character was 425 µg/L and considerable oak influence increased the aroma threshold to 569 µg/L (Figure 1). That is, the extent to which the sensory properties of a wine might be affected by 4-ethylphenol will depend on the concentration and intensity of other wine components that might mask (e.g. volatile oak compounds), or accentuate (e.g. 4-ethylguaiacol), the aroma of 4-ethylphenol.
4-Ethylphenol is produced by the enzymatic decarboxylation and reduction of p-coumaric acid (an hydroxycinnamic acid), by Dekkera/Brettanomyces sp. yeast. The hydroxycinnamates are an important group of phenolics present naturally in grapes and must, and the group include the derivatives of p-coumaric acid. p-Coumaric acid is present as the ester of tartaric acid in grapes, and postharvest hydrolysis, especially by pectin esterase, frees up the p-coumaric acid. The p-coumaric acid undergoes enzymatic decarboxylation to the corresponding vinylphenol due to the presence of cinnamate decarboxylase in yeast (not just Dekkera/Brettanomyces sp.). The last biochemcial step, the conversion of 4-vinylphenol to 4-ethylphenol, is effected by the enzyme vinyl-phenol reductase, which is present in Dekkera/Brettanomyces but is totally absent from Saccharomyces.
Although some microorganisms, such as bacteria, might be capable of volatile phenol production under some conditions (e.g. model medium enriched with certain phenols), the amounts produced are very small compared to those produced by Dekkera/Brettanomyces. Therefore, it is generally accepted that Dekkera/Brettanomyces is the only microorganism responsible for the phenol off-odour in red wines, and 4-ethylphenol is now a recognised marker compound for presence of this yeast.
4-ethylguaiacol
4-ethylguaiacol is another major spoilage compound associated with the growth of Dekkera/Brettanomyces yeast in wine and has been described as having a ‘clove’, ‘spicy’ or ‘smoky’ aroma. It is derived in same fashion as 4-ethylphenol but from a different precursor: ferulic acid. The aroma threshold for 4-ethylguaiacol is reported to be 110 µg/L, and was found to be 158 µg/L in Australian wine styles (Bramley et al. 2007). Whilst this concentration is much lower than for 4-ethylphenol, 4-ethylguaiacol is generally found in lower quantities in red wines, often 10 times less for Cabernet Sauvignon wines. Relative concentrations of 4-ethylphenol and 4-ethylguaiacol affect the overall perception of ‘Brett’ spoilage.
4-ethylcatechol
4-ethylcatechol is another spoilage compound reported to be associated with the growth of Dekkera/Brettanomyces yeast in wine and has been described as having a ‘horsey’ aroma. The aroma threshold for 4-ethylcatechol was found to be 774 µg/L in a neutral Australian red wine (Bramley et al. 2007). 4-Ethylcatechol is derived in same fashion as 4-ethylphenol but from a different precursor (caffeic acid). A survey of European wines found concentrations of 4-ethylcatechol similar to 4-ethylguaiacol (in Pinot and Cabernet wines).
Indole
Indole has been implicated in the phenomenon known as untypical (UTA) or atypical (ATA) ageing. Indole, methyl indole and aminoacetophenone, which are related to the metabolism of the amino acid tryptophan, are believed to contribute to this fault. Indole has been described as having a ‘chemical’, ‘plastic’, ‘mothballs’, ‘styrene’ and ‘rubber/plastic’ aroma. Under certain growing conditions, the grape might accumulate excessive concentrations of these compounds in the bound, glycosidic form. These bound components might later be hydrolysed, or broken, releasing the free odour-active volatiles, resulting in the fault (Zoecklein 1995). Background level in wines appear to be less than 10 µg/L. An informal sensory threshold study at the AWRI was conducted using six tasters who were sensitive to indole and an aroma threshold of 23 µg/L was obtained. Faulty wines have been observed with a concentration of indole between 30 and 350 µg/L.
Indole is also reported to be an off-flavour in beer formed by contaminant ‘coliform’ bacteria during primary fermentation. Of wines found to contain an indole fault, it is interesting to note that many have become ‘stuck’ during primary fermentation, suggesting that yeast stress might be a factor associated with the production of indole.
In recent years, indole off-flavours have been observed during the secondary fermentation of some tank-fermented sparkling wines. More information is available in this Ask the AWRI column on indole off-flavour in sparkling wine.
Wine taints:
Cork-type taints
2,4,6-trichloroanisole (TCA)
The main compound responsible for cork taint is 2,4,6-trichloroanisole. It is one of the most odour intense compounds known and has a distinct musty, mouldy aroma (Amon & Simpson 1989). One study determined the aroma threshold of TCA in a Pinot Noir wine as 1.4 ng/L (Duerr (1985), however, the aroma threshold might be lower in dry, white wines or higher in full-bodied red wines. Prescott et al. (2005) reported that the consumer rejection threshold for TCA was 3.1 ng/L whilst the consumer detection threhsold was 2.1 ng/L Some tasters at the AWRI are able to detect TCA present in wines at less that 1 ng/L. It was found by the AWRI that the presence of TCA at a concentration as low as 1 ng/L suppressed the ratings for overall aroma intensity and of positive fruit-derived characters of a particular Semillon wine during sensory evaluation.
The are a number of other odour-intense compounds present in wines that have musty, earthy or mouldy aromas and this is shown in Table 2.
Table 2. Odour-intense compounds, present in wines, imparting a musty, earthy or mouldy aromas.
| Compound |
Threshold | Odour description |
| 2-methylisoborneol | 30 ng/L | camphor-like, earthy |
| geosmin | 25 ng/L | earthy, muddy |
| 1-octen-3-ol | 20 µg/L | mushroom, metallic |
| 1-octen-3-one | 20 µg/L | mushroom, metallic |
| 2-methoxy-3,5- dimethylpyrazine |
2.1 ng/L | fungal must |
It has also been shown by the AWRI that TCA can be formed in oak, and as a result wines can have a TCA taint without any contact with cork. More information on this can be found in the ‘Ask the AWRI’ article: Wine taints from oak.
TCA is generally formed as a result of moulds growing on cork and coming into contact with trichlorophenol. Trichlorophenol and related chlorophenols are excellent biocides and have been, and appear to be still, used by industry as general disinfectants. Some moulds can detoxify the trichlorophenol and related chlorophenols by adding a methyl group to the benzene ring to form TCA and related anisoles. The chlorophenols can arise in the cork by aerial contamination or by the chlorination of phenol which is a natural component of cork.
2,4,6-Tribromoanisole (TBA)
Another compound responsible for cork taint is 2,4,6-tribromoanisole. It behaves similarly to TCA and has a similar distinct musty, mouldy aroma. Chatonnet et al. (2004) reported that the aroma of TBA was perceptible at a concentration of 4 ng/L in wine, and that it could be detected at lower concentrations by tasting. A range of exceedingly low detection thresholds in water have been reported including 8 x 10-3 ng/L (Saxby et al. 1992), 20 x 10-3 ng/L (Whitfield et al. 1997) and 30 x 10-3 ng/L (Malleret and Bruchet 2001).
Chatonnet et al. (2004) indicated that TBA can be formed in wineries by the microbial breakdown of 2,4,6-tribromophenol (TBP), which is widely used as a flame retardant and as a fungicide or wood preservative, and can also be formed in wastewater. TBA has also been detected in barrels, plastics (including synthetic closures), natural corks, wood structures including walls, floors and ceilings, and in the atmosphere of wineries. Additionally TBA is also known to be readily absorbed by plastic materials and corks.
2-Methoxy-3,5-dimethyl pyrazine (fungal must)
2-Methoxy-3,5-dimethyl pyrazine or ‘fungal must’ (FM) is possibly the second most important form of cork taint after TCA. The threshold of FM in a neutral white wine was determined to be 2.1 ng/L, which is comparable to that of TCA.
FM has been described by Mottram et al. (1984) to be responsible for an obnoxious odour present in certain machine cutting emulsions used in engineering workshops. It was described as ‘musty’, ‘foul drains’, or ‘sour dishcloths’. It has also been identified in coffee where it was described as having an earthy aroma. It was important to both raw and roasted coffee and produced an intense aroma with an aroma threshold of 0.4 ng/L in water.
Mottram et al. (1984) concluded that FM was likely to be a relatively common cause of off-odour in the environment. However, there has been no further report of it as a cause of off-odour in the published literature over the following 20 years, and only one report of its occurrence in a food product.
Chlorophenol/plastic-type taints
2,4-dichlorophenol
2,4-dichlorophenol (2,4-DCP) is one of a number of chlorophenol compounds. There are several types of chlorophenols that include mono, di, tri, tetra and pentachlorophenols and mixtures of these classes of chlorophenols have been widely used as biocides and are now considered general environmental contaminants. The aroma detection threshold of 2,4 DCP in wine was determined at the AWRI to be greater than 896 ng/L. Chlorophenols are generally described as contributing toward the odours of ‘plastic’, ‘paint’, ‘medicinal’ or ‘phenolic’.
Mono-, di- and trichlorophenols can easily be generated by the chemical chlorination of phenol. Chlorine-based sterilising agents, such as hypochlorite solutions, can react with traces of phenol present in materials such as plastic or fibreglass tanks or linings, phenolic-based resins, paints and fittings. Chlorophenols are also generated when wood is treated with hypochlorite solutions and are formed in the bleaching of wood pulp for paper manufacture. Sometimes wooden pallets loaded with cartons are stored near processing areas where disinfectants containing available chlorine are used. In situations such as this, chlorophenols can be generated in the cartons or pallets if they contact chlorine. It is interesting to note that Saxby (1992) indicates that the presence of dichlorophenols might indicate spillage of phenolic herbicides on the wooden floors of shipping containers. In addition, products such as fibreboard and paper made from recycled materials can often contain relatively high levels of chlorophenols, which can then contaminate food products or processing aids packaged in the fibreboard or paper (Mottram 1984).
2,6-Dichlorophenol
2,6-Dichlorophenol (2,6-DCP) is one of the more potent and sensorily important chlorophenol compounds which has similar aroma descriptions to general chlorophenols such as ‘plastic’, ‘paint’, ‘medicinal’ or ‘phenolic’. 2,6-DCP has an aroma detection threshold of 32 ng/L and concentrations of 2,6-DCP have been found in wines up to 236 ng/L.
2-Chloro-6-methylphenol or 6-chloro-o-cresol (6CC)
6CC has been reported to be responsible for taints in chicken meat (Patterson 1972), biscuits (Griffiths and Land 1973), soft drinks (Whitfield 1983) and more recently in wine from the use of contaminated yeast hulls. Contamination of products or processing aids with 6CC has been reported to be the result of exposure to airborne contaminants due to proximity to agrichemical plants or due to the use of disinfectants containing 6CC in the food processing plant. The AWRI determined the aroma detection threshold of 6CC in a neutral, dry white wine to be 70 ng/L.
Earthy-type taints
Geosmin
Geosmin has an ‘earthy’, ‘musty’, ‘muddy’ aroma with a sensory threshold of 25 ng/L. Both geosmin and 2-methylisoborneol are primarily known as metabolites of soil bacteria and algae, such as actinomycetes or cyanobacteria, and are responsible for off-flavours in town water supplies and fish (Young et al 1996). However, several moulds amongst those isolated from corks are also capable of their biosynthesis. Geosmin has also been reported as a metabolite of Botrytis cinerea. Although Geosmin might be present in wines, a relatively high concentration might be tolerated.
Smoke taint
When vineyards and grapes are exposed to smoke, this can result in wines with undesirable sensory characters, such as ‘smoky’, ‘burnt’, ‘ashy’ or ‘medicinal’, usually described as ‘smoke tainted’. The AWRI’s resources on smoke taint and fire damage to vineyards are available on the smoke taint page. Information on the sensory impacts of smoke taint are summarised in this fact sheet: Sensory impact of smoke exposure.
Wine flavours:
Eucalyptol or 1,8 Cineole
The ‘eucalyptus’ aroma in red wines has also been described as being ‘spicy’ and ‘mint-like’.This character in wines has typically been attributed to the monoterpene compound 1,8-cineole (1,3,3-trimethyl-2-oxabicyclo-[2.2.2]octane) which is commonly known as ‘eucalyptol’ (Farina et al. 2005). Eucalyptol has been described with terms such as ‘fresh’, ‘cool’, ‘medicinal’ and ‘camphoreous’.
There is an abundance of anecdotal evidence reporting that gum trees growing close to vineyards give off the highly volatile eucalyptus oil in warm conditions, with the oil vaporising, becoming airborne, and coming to rest on the surface of grape berries. The character is generally reported to be more pronounced in red wines because red wine fermentations are performed with the grapes in contact with their skins, and thus the oil is extracted from the skins into the wine.
Herve et al. (2003) reported that the eucalyptus aroma was discernable in red wines from California and other Mediterranean climate countries and was often suspected to be related to the presence of Eucalyptus sp. groves in the vine growing area. Eucalyptol was found at concentrations as high as 20 µg/L in wines that were considered to have a strong ‘eucalyptus’ aroma, which is much higher than the sensory threshold for this compound. The sensory detection threshold for 1,8-cineole has been reported as 1.3 µg/L in a Tannat wine sample and 1.1 µg/L in a Merlot sample (Herve et al. 2003). In order to test the theory that grapes can become aerially contaminated with eucalyptol, the authors performed a controlled trial. The results of the trial showed that eucalyptol was indeed transferred from Eucalyptus gum tree leaves to grape berries via an airborne mechanism.
Hakola et al. (2000) indicated that trees emit monoterpenes, including 1,8-cineole, in a diurnal pattern. These authors were able to measure the concentration of 1,8-cineole that was released into the air from forest plantations. This information might support the above theory that 1,8-cineole might be in higher concentrations in vines nearst to forests, such as Eucalyptus sp. plantations.
In contrast, however, it has also been reported in a paper by Farina et al. (2005) that 1,8-cineole can arise from precursors typical to the grape itself. These authors suggest that wines with a ‘eucalyptus’ character can be produced from vineyards far away from Eucalyptus tree cultivations.
Investigations into ‘eucalyptus character’, and further insight into any possible causes, however, have not as yet been conducted at the AWRI.
Rotundone is a grape-derived aroma compound that produces the distinctive ‘spicy’, ‘black pepper’ character in red grapes and wine, and most notably in cooler climate Shiraz. The compound was first identified in grapes and wine at the AWRI in 2007.
Rotundone is one of the most potent grape-derived flavour compounds, with a sensory detection threshold value of 16 ng/L in red wine. Interestingly, approximately 20% of the population is unable to smell the compound.
From a study of wines from different varieties and vintages from various regions, the majority (62%) of the wines that contained rotundone were found to be Shiraz. Perhaps not surprisingly, above aroma-threshold levels of rotundone were often encountered in wines originating from cool climate regions. Rotundone levels were also found to vary significantly from season to season, with cooler seasons generally resulting in higher levels.
Rotundone is one of the few important grape flavour compounds found free in the grape berry, rather than coming from flavourless precursors, meaning it can be tasted in grapes. Rotundone arises very late in the ripening process and is found in grape skins rather than pulp or seeds. There is evidence from commercial vineyards that clonal differences are important, but more studies are required as Australian clonal collections are generally situated in warmer climates, with negligible rotundone present.
Recent work mapped the distribution of rotundone across a Shiraz vineyard in the Grampians region of Victoria, known for producing peppery wines. Results recently published in the Australian Journal of Grape and Wine Research highlighted a surprisingly wide concentration range of rotundone in grapes across the block. The study demonstrated a very large spatial variability of the compound, and showed that it is linked to differences in soil characteristics and topography. The topographical variation pointed towards temperature and/or solar radiation effects being involved, rather than vine vigour.
An additional study has shown variation even within the grape bunch, with the most sheltered or shaded part of the bunch having the highest levels of rotundone. Assessing the effect of leaf removal, bunches that were shaded gave higher rotundone concentration than those with leaves removed from the bunch zone, further indicating the important influence of light or temperature. Similarly, higher vine vigour resulted in lower rotundone concentration.
The compound is relatively water soluble and is readily extracted from grape skins during fermentation. Studies have shown that rotundone levels in red must increase quickly early in fermentation. No yeast-related differences in rotundone levels have been observed in wine for several S. cerevisiae strains studied. Rotundone is very stable in wine with ageing, and is not removed through absorption by closures.
A component of smoke, guaiacol has been described as ‘smoky’, ‘phenolic’ and ‘medicinal’. It has a sensory threshold of 20 and 23 µg/L in white and red wine respectively, and is associated with high toast levels in oak barrels. Concentrations of 10-40 µg/L can be found in wines matured in heavily toasted oak.
4 methylguaiacol also contributes toward ‘smoky’ characters in wine and has been described as having a ‘char’ and ‘spicy’ aroma. It is formed almost exclusively during the process of toasting oak barrels (Godden et al. 1999). Boidron et al. (1988) reported the sensory threshold of 4-methylguaiacol in red wine as 65 µg/L.
Guaiacol, along with other oak volatiles, increases during the barrel toasting process. Table 3, which is adapted from Ribéreau-Gayon et al. (2006), shows the impact of toasting intensity on the formation of various volatile phenol compounds found in oak. These volatile phenols can be extracted from oak into wine during storage and their composition reflects the structure of the lignin in the particular oak, as well as the heating temperature. It can be seen from the table that the syringol derivatives extracted increase dramatically in the toasted oak compared to the non-toasted oak. Guaiacol and 4-methyl guaiacol were also extracted from the toasted oak at significant levels. Note, however, that very little of the cresol compounds was extracted, even from the heavily toasted oak.
| Oak volatile phenol | Units | Non- toasted | Light | Medium | Heavy |
| Guaiacol | µg/L | 1 | 5.2 | 27.7 | 30.3 |
| Methyl-4-guaiacol | µg/L | 2 | 10 | 38.7 | 24.7 |
| Ethyl-4-guaiacol | µg/L | 0 | 0 | 0 | 7.7 |
| Propyl-4-guaiacol | µg/L | 0 | 0 | 0 | 6.3 |
| Eugenol | µg/L | 20 | 17.7 | 71.7 | 44.3 |
| Phenol | µg/L | 5 | 12 | 11.7 | 20 |
| Ortho-Cresol | µg/L | 0 | 0 | 0 | 1.7 |
| Meta-Cresol | µg/L | 0 | 0 | 0 | 1.3 |
| Para-Cresol | µg/L | 0 | 0 | 0 | 2 |
| Syringol | µg/L | 0 | 78.3 | 310.7 | 313.3 |
| Methyl-4-syringol | µg/L | 0 | 17.3 | 80.7 | 193.3 |
| Allyl-4-syringol | µg/L | 0 | 60.3 | 298.7 | 204.3 |
Results are a mean of several analyses, compounds extracted in a dilute alcohol medium, under standard conditions.
REFERENCES
Amon, J.M., Vandepeer, J.M., Simpson, R.F. 1989. Compounds responsible for cork taint in wine. Aust. N.Z. Wine Ind. J. 4(1): 62-69.
The Australia and New Zealand Food Standards Code
Chatonnet, P., Dubourdieu, D., Boidron, J.N., Pons, M. 1992. The origin of ethylphenols in wines. J. Sci. Food Agric. 60: 165-178.
Chatonnet, P., Dubourdieu, D., Boidron, J.N., Lavigne, V. 1993. Synthesis of volatile phenols by Saccharomyces cerevisiae in wines. J. Sci. Food Agric. 62: 191-202.
Chatonnet, P., Bonnet, S., Boutou, S., Labadie, M.-D. 2004. Identification and responsibility of 2,4,6-tribromoanisole in musty, corked odors in wine. J. Agric. Food Chem. 52: 1255-1262.
Chisholm, M.G., Samuels, J.M. 1992. Determination of the impact of the metabolites of sorbic acid on the odor of a spoiled red wine. J. Agric. Food Chem. 40(4): 630-633.
Costello, P.J., Lee, T.H., Henschke, P.A. 2001. Ability of lactic acid bacteria to produce N heterocycles causing mousy off-flavour in wine. Aust. J. Grape Wine Res. 7(3): 160-167.
Coulter, A. 2023. Ask the AWRI: Wine taints from oak. Aust. N.Z. Grapegrower Winemaker (709): 56-57.
Crowell, E.A., Guymon, J.F. 1975. Wine constituents arising from sorbic acid addition, and identification of 2-ethoxyhexa-3,5-diene as source of geranium-like off-odor. Am. J. Enol. Vitic. 26(2): 97–102.
Curtin, C., Bramley, B., Cowey, G., Holdstock, M., Kennedy, E., Lattey, K., Coulter, A., Henschke, P., Francis, L., Godden, P. 2008. Sensory perceptions of ‘Brett’ and relationship to consumer preference. Blair, R.J., Williams, P.J., Pretorius, I.S. (eds) Proceedings of the thirteenth Australian wine industry technical conference, 29 July – 2 August 2007, Adelaide, SA: Australian Wine Industry Technical Conference Inc.: Adelaide, SA: 207-211.
Duerr, P. 1985. Wine quality evaluation. Proceedings of the international symposium on cool climate viticulture and enology. 25-28 June 1985, Eugene, OR & Corvallis, OR: Oregon State University: 257-266.
Edinger, W.D., Splittstoesser, D.F. 1986. Production by lactic acid bacteria of sorbic alcohol, the precursor of the geranium odor compound. Am. J. Enol. Vitic. 37(1): 34−38.
Farina, L., Boido, E., Carrau, F., Dellacassa, E. 2005. Terpene compounds as possible precursors of 1,8-cineole in red grapes and wines. J. Agric. Food Chem. 53: 1633-1636.
Godden, P.W. 2002. Update on the AWRI trial of the technical performance of various types of wine bottle closure. AWRI Tech. Rev. 139: 610.
Grbin, P.R., Costello, P.J., Herderich, M., Markides, A.J., Henschke, P.A., Lee, T.H. 1996. Developments in the sensory, chemical and microbiological basis of mousy taint in wine. Stockley, C.S., Sas, A.N., Johnstone, R.S., Lee, T.H. (eds) Maintaining the competitive edge: proceedings of the ninth Australian wine industry technical conference, 16-19 July 1995, Adelaide, SA: Winetitles, Adelaide, SA: 57-61.
Griffiths, N.M., Land, D.G.1973. 6 chloro o cresol taint in biscuits. Chem. Ind. 904.
Hakola, H., Laurila, T., Rinne, J., Puhto, K. 2000. The ambient concentrations of biogenic hydrocarbons at a northern European, Boreal site. Atmosph. Environ. 34: 4971-4982.
Herve, E., Price, S., Burns, G. 2003. Eucalyptol in wines showing a Eucalyptus aroma. Poster paper. Actualities Oenologiques 2003 VIIme Symposium International d’Oenologie, 19-21 June 2003, Bordeaux, France.
Malleret, L., Bruchet, A. 2001. Application of large volume injection GC/MS to the picogram analysis of chlorinated and brominated anisoles in earthy-musty off-flavor water samples. Water Sci. 1: 1-8.
Mottram, D.S., Patterson, R.L.S., Warrilow, E. 1984. 2,6-Dimethyl-3-methoxypyrazine: a microbiologically-produced compound with an obnoxious musty odour. Chem. Ind. 448-449.
Patterson, R.L.S. 1972. Disinfectant taint in poultry. Chem. Ind. 609-610.
Prescott, J., Norris, L., Kunst, M., Kim, S. 2005. Estimating a ‘consumer rejection threshold’ for cork taint in white wine. Food Qual. Pref. 16(4): 345-349.
Ribéreau-Gayon, P., Dubourdieu, D., Donèche, B., Lonvaud, A. 2006. Handbook of enology. Volume 1: the microbiology of wine and vinifications. Chichester, UK: John Wiley & Sons: xiv, 497.
Saxby, M.J., Reid, M.J., Wragg, G.S. 1992. Index of Chemical Taints. Leatherhead Food RA: Leatherhead, UK.
Simpson, R.F. 1990. Cork taint in wine: a review of the causes. Aust. N.Z. Wine Ind. J. 5(4): 286-287, 289, 291, 293-296.
Sneyd, T.N., Leske, P.A., Dunsford, P.A. 1993. How much sulfur? Stockley, C.S., Johnstone, R.S., Leske, P.A., Lee, T.H. (eds) Proceedings of the eighth Australian Wine Industry Technical Conference, 25-29 October 1992, Melbourne, Vic. Winetitles, Adelaide, SA: 161-166.
Whitfield, F.B., Hill, J.L., Shaw, K.J. 1997. 2,4,6-tribromoanisole: a potential cause of mustiness in packaged food. J. Agric. Food Chem. 45: 889-893.
Young, W.F., Horth, H., Crane, R., Ogden, T., Arnott, M. 1996. Taste and odour threshold concentrations of potential potable water contaminants. Wat. Res. Vol. 30(2): 331-340.
Zoecklein, B.W., Fugelsang, K.C., Gump, B.H., Nury, F.S. 1995. Wine analysis and production. New York: Chapman and Hall.

