Technical Review No. 260, October 2022
Marlize Bekker, Principal Research Scientist, marlize.bekker@awri.com.au
Allie Kulcsar, Scientist
Lisa Pisaniello, Scientist
Flynn Watson, Senior Scientist
Tracey Siebert, Research Scientist
Toni Garcia Cordente, Research Scientist
Introduction
Phenylmethanethiol (PMT), also known as benzylmercaptan, is a sulfur compound associated with ‘struck flint’ aroma in wine. This character is considered desirable in certain styles of premium Chardonnay and sparkling wine; and while some winemakers have empirical knowledge on how to express it in their wines, little scientific evidence has been gained to describe its formation or winemaking techniques that could be used to dial this character up or down.
As part of a broader Wine Australia-funded project on sulfur compounds in wine, AWRI researchers conducted experiments to investigate the formation of this compound and factors influencing its preservation in wine during ageing. The key research questions investigated in these experiments were:
- Which winemaking techniques can be implemented to increase and control the concentration of PMT?
- What can winemakers do to preserve ‘struck flint’ aroma in ageing wine?
- How do PMT concentrations evolve in ageing wine?
Investigating factors affecting PMT formation
Experiments were conducted in model wine containing benzaldehyde (5 μM) and hydrogen sulfide (H2S, 5 μM), which are known to be precursors of PMT. The influences of pH, oxygen, copper (0.5 mg/L) and iron (4 mg/L) on the formation of PMT from these precursors were then evaluated over a 17-month period. Results from this study are presented in Figure 1.
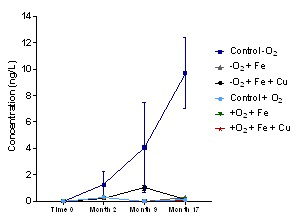
Figure 1. Results from experiments evaluating factors influencing PMT formation from its precursor compounds, benzaldehyde and hydrogen sulfide, in model wine
The first key finding from this work was that no PMT was produced in any of the samples that were exposed to oxygen. This is likely due to the precursor compound hydrogen sulfide being oxidised and therefore not available to form PMT. Secondly, no PMT was formed in samples treated with copper and/or iron. This is believed to be due to the complexation of hydrogen sulfide with the copper or iron ions, again leaving it unavailable to react with benzaldehyde to form PMT. In this experiment, PMT was only produced in model wine (pH 3) that had been protected from oxygen and did not contain added metals. No PMT formation was observed at pH 4 under any experimental condition.
Investigating the preservation of PMT
Further experiments were conducted in model wine to evaluate the effects of copper (0.5 mg/L), iron (4 mg/L), oxygen, and elevated residual concentrations of H2S (10 and 40 μg/L) and sulfur dioxide (SO2, 20 and 40 mg/L) on the preservation of PMT over a six-month period. Results from this series of experiments are presented in Figure 2.
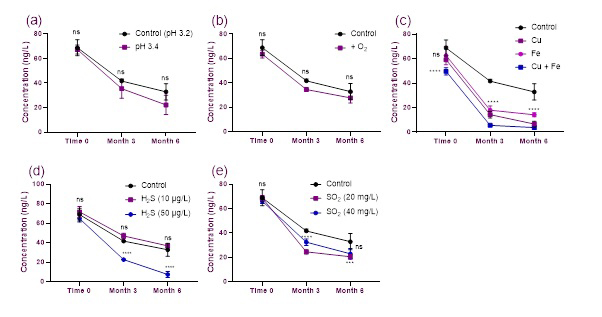
Figure 2. Evaluating factors that influence PMT preservation in model wine. Error bars indicate standard deviation for n =3 samples. Significant interactions are indicated as follows: not significant (ns) for p value > 0.05; * p value ≤ 0.05; ** p value ≤ 0.01; *** p value ≤ 0.001; **** p value ≤ 0.0001.
The concentration of PMT was found to decrease in all samples over the six-month period. No effect on PMT concentration was seen when pH was varied from 3.2 to 3.4, while exposure to oxygen resulted in a slight decrease in PMT concentrations (Figure 2b). The presence of copper and iron caused significant decreases in PMT concentrations, with the combination of copper and iron having the most detrimental effect on PMT preservation (Figure 2c). High concentrations of H2S and SO2 also significantly decreased PMT over time (Figures 2d and 2e).
Evolution of PMT in ageing wines
As the third part of this work on PMT, the evolution of PMT in 10 commercially available Chardonnay wines from Australia and New Zealand from the 2018 and 2019 vintages was studied over a six-month period. Concentrations of PMT decreased in all 10 wines after six months (Figure 3), but the rate of decrease was unique to each wine. This suggests that there may be many factors that influence the rate of PMT degradation in wine.
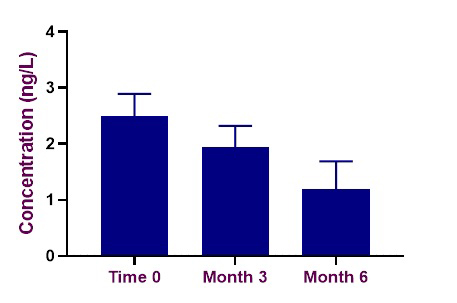
Figure 3. The average concentration of PMT measured in 10 Chardonnay wines over the course of six months
Conclusions and future work
The results from this initial study on the formation, evolution and preservation of PMT suggest it is likely that winemaking interventions will be able to modulate the concentration of this flavour compound. Key findings included:
- Anaerobic conditions promoted the formation of PMT from hydrogen sulfide and benzaldehyde in model wine.
- Metal ions both inhibited the formation of PMT and had detrimental effects on its preservation in model wine.
- The concentration of PMT concentration naturally decreased in ageing Chardonnay wines, albeit at different rates for individual wines.
Additional work in progress on this important compound is extending these initial model studies into real wine systems, including assessment of the influence of yeast strain selection and must nitrogen content on PMT production.
Acknowledgements
This work is supported by Australia’s grapegrowers and winemakers through their investment body, Wine Australia, with matching funding from the Australian Government. The AWRI is a member of the Wine Innovation Cluster in Adelaide, SA.

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Recommended attribution:
Bekker, M., Kulcsar, A., Pisaniello, L., Watson, F., Siebert, T., Garcia Cordente, T. Technical Note – first steps in understanding the formation and preservation of ‘struck flint’ aroma in wine. AWRI Technical Review No. 260, October 2022. https://www.awri.com.au/tr/tech-note/struck-flint-aroma-in-wine/

