Aim
To count the total number of cells (viable and non-viable) in culture. Methylene blue is added to the yeast sample, and the non-viable cells will be stained blue.
Safety issues
Methylene blue is toxic. Wear gloves and glasses.
Time frame of procedure
Assuming you already have a broth culture and stock solutions it will only take about 20 minutes per flask.
Equipment
- Microscope
- Haemacytometer & cover slip
- Pipetter & tips
- Counter
- Tubes
Materials & reagents
Methylene blue solution: Dissolve 0.01 g methylene blue in 10 mL of distilled water. Dissolve 2 g sodium citrate dihydrate in the mixture with stirring. Filter through a filter paper and make volume of the filtrate to 100 mL with distilled water.
Procedure
Dilution
- Dilute the culture in saline, peptone, or sterile water. Try 1:100 (i.e. 0.1 mL + 9.9 mL)
- Mix thoroughly.
- To 0.9 mL of the diluted yeast add 0.1 mL of the methylene blue solution, mix and stand for 10 minutes.
Total count
- Mix thoroughly and place an amount on the haemacytometer, cover with the cover slip and count under the microscope using 40X magnification. Remember to remove the green glass filter from the light source.
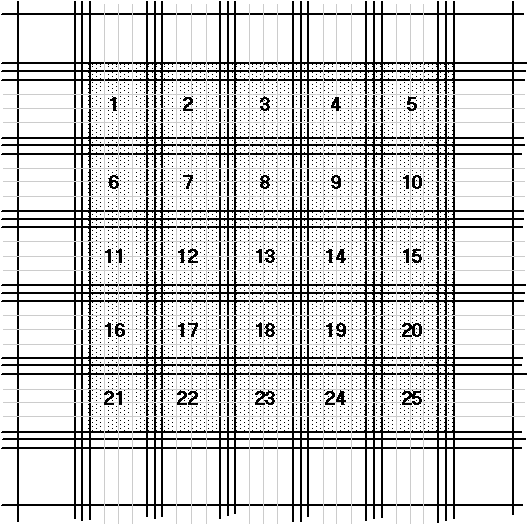
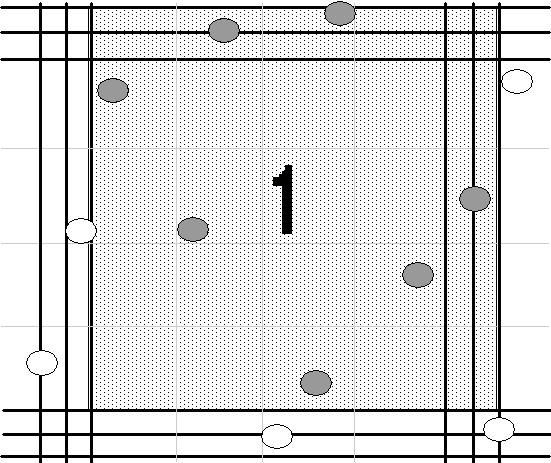
- Count all the cells in the shaded area as described in Figure 1. Figure 2 shows an enlargement of square number 1.
- Record the number and count the next area. There are two countable areas on the haemacytometer.
- Budding cells – If the bud is > 1/3 the size of the ‘mother’ cell, count it. If it is
- You should aim to count between at least 600 cells in total. You may need to place another sample on the haemacytometer to obtain counts over four countable areas.
Non-Viable count
- Using the same view under the microscope, count the blue/purplish cells in the same area.
- Record the number and repeat for the second area.
Clean up
- Carefully remove the cover slip. Gently wipe the cover slip and haemacytometer with a 70% alcohol soaked tissue.
- Replace it the box provided.
- Replace the green glass and turn the microscope off and cover it.
- Discard all tips in an autoclave bag for disposal.
Calculation of results and data interpretation
- The blue coloured cells are the non-viable cells, and the colourless cells are viable. A total count is the viable and non-viable cells.
- Average the number of cells over the two countable areas.
- Yeast cell concentration (total cells/ml) calculation is as follows:
Your averaged count of 25 squares x 104 x dilution x 1.11 (dilution of staining) - If you counted only 5 squares of the total 25 squares, multiply by 5.
Total count = Average count (5 squares) x dilution x 104 x 1.11 x 5 = cells / mL
Trouble Shooting
- If there are too many cells to count make a larger dilution, ie 1:1000 in step 1 of the dilution stage. If you have kept the 1:100 dilution, make a 1:10 dilution of this and repeat the methylene blue steps.
- Alternatively, you can count five squares of the 25 as described in Figure 1 and multiply the count by 5.
- If there are not enough cells you will need to make a smaller dilution, ie 1:10 or even neat if required to obtain an accurate count.
- If the cells do not appear blue, check you have incubated for 10 minutes.
- If all the cells appear blue, check you have not incubated the cells for longer than 10 minutes. This will also happen if the culture is basically dead.
- If the cells appear green, check you have removed the green glass.
 |
|
| Count these as two. | Count this as one. |

