Gas adjustments at bottling
When preparing a wine for bottling, a wine may be moved from tank to tank during stabilisation, filtration and/or clarification processes. The large surface area of the wine exposed during these wine movements, and the inability to keep effective inert gas coverage, results in large amounts of gas exchange, resulting in more oxygen dissolving into the wine. At this point in its life a wine can contain up to 4 mg/L of oxygen. During packaging, the wine can also be exposed to additional oxygen from the closure or headspace of the wine, even with effective inert gas coverage techniques.
As 1 mg/L of oxygen can indirectly result in the loss of up to 4 mg/L free sulfur dioxide, it is important to minimise both the dissolved and headspace oxygen in the wine pre-packaging to ensure adequate levels of sulfur dioxide are retained in the wine after bottling. The dissolved oxygen levels in wine are often reduced to acceptable levels before bottling (generally < 0.5 mg/L) using inert gas sparging techniques.
Sparging operation
Sparging involves very fine bubbles of an inert gas being bubbled through the wine. Wineries often have reticulated gas systems rather than having gas cylinders directly connected to tanks. Wines are sparged with gas either via a gas line connection to a valve at the bottom of a tank, via a submergible bubbler or in-line during a wine transfer between tanks or during pump-around of a tank. The gas-liquid interface is critical in sparging wine and a high surface contact area can be achieved (>100,000 m2 per m3) by dispersing very fine bubbles of gas throughout a liquid. The small bubble size is achieved using stainless steel sinters connected to the gas line. Sinters vary from 2 to 100 µm, but the most common sinter porosity is around 15 µm. Smaller sinters can be more efficient but can also block more easily and/or limit gas flow rate. Dose rates range from 0.1 to 0.3 litres of gas per litre of wine. Sparge or contact time is generally around 30 seconds. After sparging, the dissolved oxygen is measured and the process continued until the desired dissolved oxygen level has been achieved. Sparging, while effective at removing oxygen, could potentially remove some positive volatile aroma compounds. Thus effective inert gas coverage during storage and previous wine movements is important to limit the amount of dissolved oxygen and thus sparging required.


White table wines are generally sparged to reduce dissolved carbon dioxide until they contain between 0.8 and 1.3 g/L. Levels below this can make a wine appear flat. With levels higher than this, the wine can taste spritzy. Red table wines are generally sparged until they contain <0.5 g/L of dissolved carbon dioxide. Excess levels can give red wines a hardness on the palate by enhancing perceived acidity, tannin and bitterness and reducing perceived sweetness.
Sparging and blanketing gases
The main gases used for sparging are nitrogen and carbon dioxide, or combinations, supplied either in bulk liquid form or in cylinders. Argon has occasionally also been used for gas blanketing of tank head space. For information on gases used to protect wine from air/oxygen in tanks during wine storage, or blanketing gases, see Gases – we have you covered (PDF article). Gases should be food grade and purity should be 99.5%.
Gases are less soluble in wine than in juice or water due to the alcohol concentration. Gases are also more soluble at lower temperatures. The saturation point of oxygen in wine is 8 mg/L at 20ºC but its solubility increases to 14 mg/L in wine held at 0ºC. Thus, holding wines cold pre-bottling may result in greater levels of dissolved oxygen. However, as oxidation reactions are slower at lower temperatures, many bottlers use a compromise sparging and bottling temperature of around 15°C. Lower bottling temperatures also make label application and adherence more difficult.
| Solubility of oxygen, nitrogen and carbon dioxide in wine at room temperature | ||
|---|---|---|
| Gas | Unit | Solubility in wine at 20ºC |
| Oxygen | mg/L | 8 |
| Carbon dioxide | mg/L | 1,500 |
| Nitrogen | mg/L | 14 |
Note: gases have different solubilities in water, juice and wine, with solubility varying with temperature, pressure, alcoholic strength, sugars and polysaccharides and phenols.
How does sparging work?
Sparging introduces bubbles into wine. Gas exchange occurs at the surface of the bubbles and the bubbles then leave out the top of the tank. For example, in the case of sparging using nitrogen, there is more carbon dioxide and oxygen in the wine than in the nitrogen bubbles, so some carbon dioxide and oxygen transfer into the nitrogen bubbles, and are removed from the wine.
Turbulence increases contact of the wine with the sparging gas and thus increases sparging efficiency and can decrease the sparge time required. Sparging via a T-piece, or a 90 degree angle, or a spiral or vortex sparger in-line can increase effectiveness (Figure 2). Automated membrane contactors for in-line dissolved gas management have also been more adopted by some wineries (see AWRI webinar and associated articles). Similar gas exchange principles apply, except that gas exchange occurs in the pores of a membrane instead of on the surface of bubbles. Wine flows on one side of the membrane and a gas or vacuum flows on the other side. Only gases diffuse through the small pores in the hydrophobic membrane. Transfer is bubble-less.
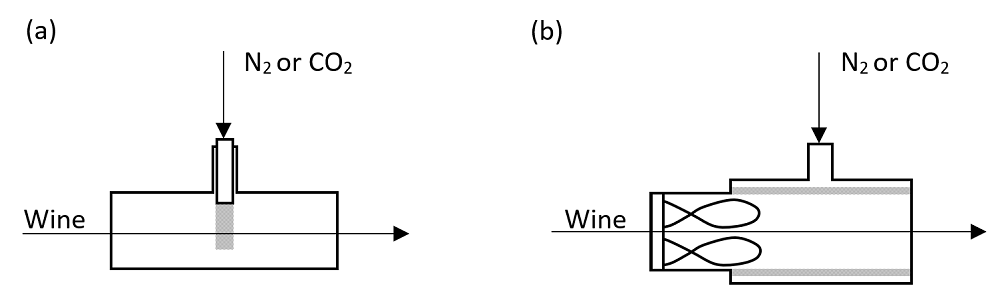
Figure 2. In-line spargers (a) standard and (b) with elements to promote turbulence. Adapted from: Nordestgaard, S. Gains in speed, labour and gas consumption for winemakers. Aust. N.Z. Grapegrower Winemaker (648): 61-67; 2018 – full text available
For red wines where dissolved carbon dioxide is not desired, nitrogen gas can be used alone as the sparging gas, due to its lower solubility in wine. Carbon dioxide gas can be used; however, back sparging with nitrogen gas is then needed to lower the dissolved carbon dioxide concentration. Alternatively, mixtures of carbon dioxide and nitrogen can be used simultaneously.
Measurement of oxygen
The measurement of dissolved oxygen (DO) in finished wines is not new to the wine industry and several types of DO meter have been available for some time. There are two main types of oxygen sensor – electrochemical and optical. Electrochemical cells have been used since the 1950s – originally for measuring oxygen in blood. Orbisphere is a brand using an electrochemical cell that has been common in the wine industry. Optical DO sensors are becoming more popular because they are more robust and repeatable.
Instruments such as the Anton Paar CboxQC™ and Orbisphere 3650 can be found in bottling laboratories. Handheld meters, such as the Hach HQ, can be used to measure tank DO prior to bottling or even headspace oxygen during ullage management. Depending on the model, handheld devices should be frequently calibrated, preferably using a two-point calibration. Another DO-measuring technology uses luminescence and is often referred to as LDO – this is the system used in the AWRI bottling line oxygen audits.
Robust DO probes are available which are better suited for use on the cellar floor, particularly when built into pump-over rigs. The AWRI has been using stainless steel probes – routinely used in the brewing industry – with a stand-alone monitor mounted in 2-inch and 3-inch fittings. This set-up has enabled the determination of DO during juice and wine transfers and in large-scale aeration trials with a commercial venturi. Another innovative device that has been trialled is a small DO datalogger (operated on two AA batteries with a standard digital camera memory card) which can be deployed in a tank and left to monitor DO over several hours, days or weeks before recovery and data download. With this device it has been possible to monitor the DO inside a press during both inert and normal aerobic press cycles.
References and reading list
- Allen, D.B. 1990. New developments in sparging technology. Williams, P.J., Davidson, D.M., Lee, T.H. (eds.) Proceedings of the seventh Australian Wine Industry Technical Conference, Adelaide, SA., 13-17 August 1989, Adelaide, S.A.; Australian Wine Research Institute: 266-267.
- Allen, D.B. 1991. A new twist in sparging. Aust. Grapegrower Winemaker (334): 55-57.
- Boulton, R.B., Singleton, V.L., Bisson, L.F., Kunkee, R.E. 1996. Principles and practices of winemaking. New York: Chapman & Hall: xiv, 604, 432-434.
- Cordingley, B. 2023. Ask the AWRI: Gases – we have you covered. Aust. N.Z. Grapegrower Winemaker (716): 68-69.
- Date, S. 1990. Mathematical model for CO2 stripping using sparging. Williams, P.J., Davidson, D.M., Lee, T.H. (eds.) Proceedings of the seventh Australian Wine Industry Technical Conference, Adelaide, SA., 13-17 August 1989, Adelaide, SA.; Australian Wine Research Institute: 268-269.
- Day. M. 2014. Introducing and measuring oxygen during active ferments. AWRI Tech. Rev. 213: 6-10.
- Nordestgaard, S. 2018. Gains in speed, labour and gas consumption for winemakers. Aust. N.Z. Grapegrower Winemaker. 648,: 61-67.
- Zoecklein, B.W., Fugelsang, K.C., Gump, B.H., Nury, F.S. 1995. Wine analysis and production. New York: Chapman & Hall: xviii, 621; 222-225.

